User Acceptance Testing for Clinical Trials
User Acceptance Testing (UAT) seamlessly executed by unbiased, independent experts. Streamline this necessary but challenging and resource-intensive milestone on the path to first patient in.

Keep your study on track and on budget
We’ve honed our technical expertise over 15 years and 2,000+ clinical trials (eCOA/ePRO/EDC). Studion can help you avoid delays, meet regulatory rigor, minimize software amendments, and reduce strain on internal teams.
UAT Services
We offer a full-service User Acceptance Testing solution. Contact us to customize a plan to fit your needs.
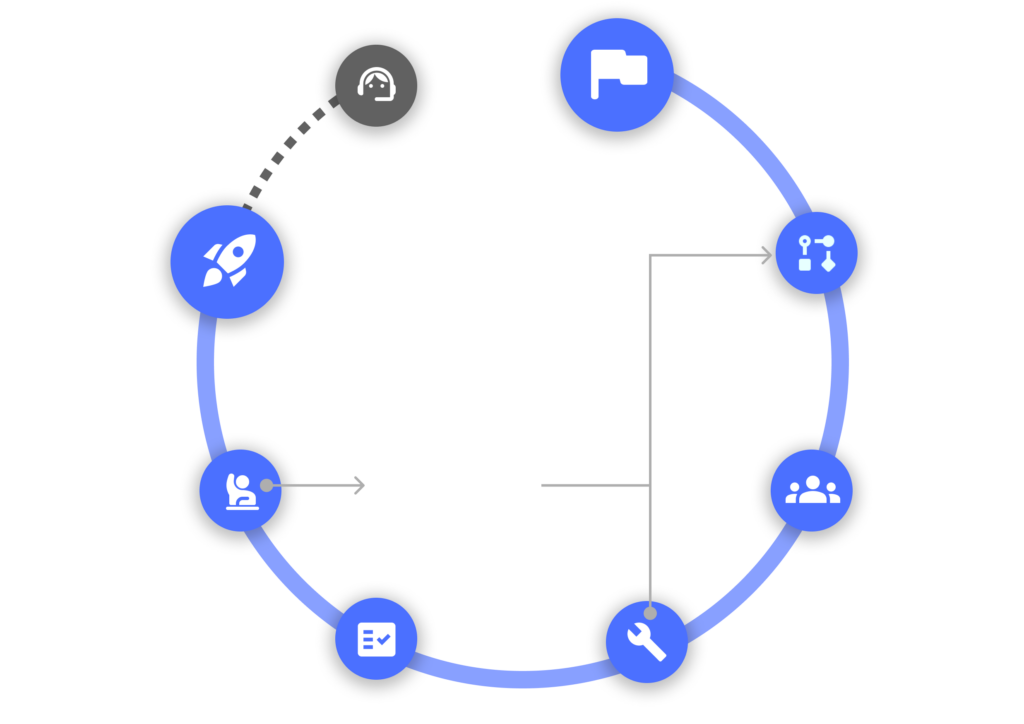
How we deliver results
Our dedicated team takes a holistic approach to provide unparalleled ease and efficiency and ensure your system meets the needs of the study protocol.

Objective Review
- Independent 3rd party review removes any conflict of interest
- Fresh perspective and experience to identify potential designs that may impact patient engagement.

Collaborative and Adaptable Approach
- Understand your needs and goals when shaping a comprehensive UAT plan
- Update and adapt test scripts as needed
- Ongoing support throughout the testing process
Clearly Defined Process
- Define the UAT process and protocols at the beginning
- Maximize efficiency by foreseeing obstacles
- Identify key design gaps as early as possible
- Ensure interoperability of systems representing the end user experience

Regulatory Compliance
- Strict adherence to guidelines and key protocol requirements
- Deep knowledge of regulatory requirements
- Prioritize data security
Our Experience

0+
Trials

0+
Therapeutic Areas

0+
Platforms

0+
Years